Rust is the natural state of iron (ferrous oxide). Rust will occur when an iron containing metal alloy, such as steel, drops below a pH of 8.2 or 8.3. There are rust inhibitors that can combat the pH drop on the surface, preserving the alloy under the surface.
You can tell when the pool light screw goes missing and someone assumes any screw can be put in it’s place. The result is a rusted “regular” screw within hours or days. Iron left exposed to air will corrode and form a flaky orange-red outer layer.
Salt Water Pools – Why do they have the potential to cause issues with corrosion/rust of metals?
When you add salt to water, it speeds up the process of the reaction between the water and metal because it makes the water more conductive. By increasing the concentration of ions in the water (from the salt, NaCl) the rate of oxidation/corrosion of metal increases.
Corrosion Factors?
A measure of acidity or alkalinity of water soluble substances (pH stands for ‘potential of Hydrogen’).
The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. A pH greater than 7 is basic.
Water with a pH below 6.5 will be corrosive, especially if alkalinity also is low. However, water with pHvalues above 7.5 also can be corrosive when alkalinity is low. Minerals dissolved in water separate into charged particles (ions) that conduct electricity. Corrosion causes both pinhole leaks and stress cracking in metal components.
What happens if you swim in a pool with low pH?
Corrosion Resistant Metals:
- Galvanized Steel. Galvanized steel is the least corrosion resistant metal on this list.
- Aluminum. One step above galvanized steel is aluminum.
- Copper, Brass, Bronze, or naval bronze follow. Brass and Bronze have a relatively high zinc content. They are not recommended for plumbing applications below water or in saltwater situations.
- Copper does not rust – as rust is iron oxide and only corrosion of iron can create rust. Copper and Bronze cannot rust, but they can corrode or tarnish.Do not let the copper dry on its own when it is wet because this will increase the potential of tarnish forming. When there is prolonged contact with human skin and it can react with air, sweat, chemicals, lotions and makeup, copper can turn green or blue green and stain the skin. The green patina that forms naturally on copper and bronze, sometimes called verdigris, usually consists of varying mixtures of copper chlorides, sulfides, sulfates and carbonates, depending upon environmental conditions such as sulfur-containing acid rain. Oxidization, a chemical reaction between copper and oxygen, can turn acopper pot black. When heated, copper can develop a layer of brownish-black copper oxide. If it is not polished away, the copper oxide will react with the air’s moisture to form copper carbonate, which is green in color.
- Stainless Steel is a widely used metal for swimming pool metal screws and components.
- Stainless steel contains significant amounts of chromium. When exposed to the atmosphere the surface oxidizes slightly and a thin film of chromium oxide forms, preventing any further oxidation. If exposed to water, salt or fresh, without the presence of air, this film will not form and the metal will corrode.
- Copper, Palladium, Silver, Platinum and Gold are Noble Metals – and don’t corrode easily which is why they are used in jewelry and coins.
What’s the difference between 304 and 316 stainless steel?
- 304 contains 18% chromium and 8% nickel
- 316 contains 16% chromium, 10% nickel and 2% molybdenum. The molybdenum is added to help resist corrosion to chlorides (like sea water and de-icing salts).
Alloy 316/316L is molybdenum-bearing austenitic stainless steel and has better corrosion resistance.
Stainless steel doesn’t rust because it is an alloy that contains the metals chromium and nickel. Chromium must comprise at least 10 percent of the steel alloy for stainless steel to be rust resistant. The chromium in stainless steel when exposed to oxygen in the atmosphere forms a thin invisible layer called chromium oxide. This invisible layer covering the entire surface gives stainless steel its ability to resist stains and rust. If this layer is damaged rust is formed on the surface at the point of that damage.
Corrosive Water
Water becomes more corrosive when:
- Bacteria level in water (bacteria such as sulphur reducing bacteria (SRB) increase the corrosivity level of water
- Chlorine Levels – Higher Chlorine levels make water more corrosive
- Conductivity of water – higher electrical conductivity makes water more corrosive, some dissolved solids or metals increase conductivity
- Solids Suspended – Sand, sediment or even moving rust particles in high flowing water can be corrosive
- Water Flow Rate – the faster the flow rate, corrosive effects are increased
- Water Temperature – higher temperatures mean more corrosive water
- Water pH – water below 6.5 or above 8.5 – low pH acidic water is corrosive, high pH water alkaline is scale forming
- Gasses dissolved in water – higher levels of oxygen or carbon dioxide for example increase water corrosivity
Failures not due to pitting corrosion (i.e. caused by erosion and corrosion or corrosion fatigue) occurred in waters with a higher pH and higher HCO3- content
Well Water
Source Water: acidity is a natural condition of some well water that is a nuisance but generally not a health hazard. Water with a pH below 7.0 is considered acidic; above 7.0 it is basic or alkaline. The U.S. EPA doesn’t regulate the pH level in drinking water, but it recommends levels from 6.5 to 8.5.
Langlier Saturation Index LSI
The Langelier index is calculated using pH, temperature, total dissolved solids, and total hardness. The LI is a measure of the balance of pH and calcium carbonate (CaCO3)
As the LI value becomes more positive the water is more saturated with CaCO3 which results in precipation – this can coat and protect pipes but can cause scaling as well. Langelier, who developed this index, realized that the acidity or pH of water determines how much calcium carbonate CACO3 the water can hold.
The Langelier calculation factors in the main components of corrosivity of a water supply including:
- Temperature of the water
- pH of the water
- total dissolved solids or TDS in mg/L
- Calcium level Ca in mg/L and whether or not the Ca is in the form of calcium carbonate CaCO3 or calcium ions Ca2+
- Alkalinity of the water (in mg/L as calcium carbonate) these produce a Langelier Saturation Index.
The Langelier Index (LI), when calculated ranges from +4 through 0 to -5. Water with an LI above 0.5 (tending to form scale) or with an LI below -0.5 (tending to corrode metal pipes, tanks, etc), probably needs treatment.
+4 LSI = scale producing water
Zero LSI = Neutral – not scale producing not acidic
At -5 LSI = very corrosive water
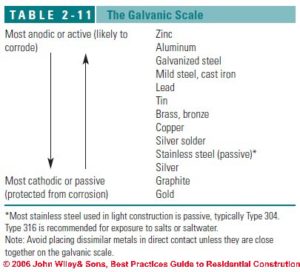
Galvanic corrosion
Galvanic corrosion is deterioration of metal caused by an electrochemical reaction that happens when two metals are in contact with one another – commonly underwater or wet with a liquid that acts as an electrolyte. The galvanic corrosion occurs around the point of contact between the two metals. Salts that increase the conductivity of the water or moisture increase the galvanic corrosion activity.
Galvanic corrosion can occur at both metals through the action of an electrical current (galvanic current) that occurs at the anodic and at the cathodic member of the pair of metals. The anode member of the pair of metals gives up metal as ions of that metal move to and are deposited on the cathode member of the pair.
Bonding or Grounding
Bonding: all metallic parts are permanently joined to form an electrically conductive path to reliably conduct electrical current.
Grounding: Connection of an electrical circuit or component to the earth.